Once-weekly dulaglutide compared with daily insulin glargine as basal therapy,
both in combination with insulin lispro1
Despite increasing awareness and new developments in the treatment and prevention of diabetes, there has been a rapid increase in the number of people with T2D.²
Tight glycemic control may reduce the risk of diabetes complications in people with T2D.³Non-adherence to treatment is a barrier to effective care. Only 50% adherence to long-term therapy exists among patients with chronic diseases in developed* countries.4*The magnitude and impact of poor adherence in developing countries is assumed to be even higher.
Additionally, clinical inertia can be encountered at any step in T2D management and can delay appropriate treatment intensification for both oral anti-diabetic agents and injectable therapy.5
AWARD: A clinical trial programme spanning the continuum of care
AWARD 36
Dulaglutide vs metformin
Drug-naive or washout from 1 OAM
AWARD 57
Dulaglutide vs sitagliptin
Add-on to metformin
AWARD 68
Dulaglutide vs liraglutide
Add-on to metforminAWARD 89
Dulaglutide vs placebo
Add-on to glimepiride
AWARD 110
Dulaglutide vs exenatide
Add-on to metformin and pioglitazoneAWARD 211
Dulaglutide vs insulin glargine
Add-on to metformin and glimepirideAWARD 1012
Dulaglutide vs placebo
Add-on to SGLT2 inhibitors with or without metformin
AWARD 413
Dulaglutide vs insulin glargine
Both with mealtime insulin lispro
with or without metformin
AWARD 914
Dulaglutide vs placebo with insulin glargine
with or without metforminAWARD 71
Dulaglutide vs insulin glargine
Add-on to prandial lisproAlmost 40% of patients with T2D will likely develop moderate renal impairment and approximately 2% to 3% of them will progress to severe renal impairment.15

AWARD-7 Trial Summary
Research Design and Method
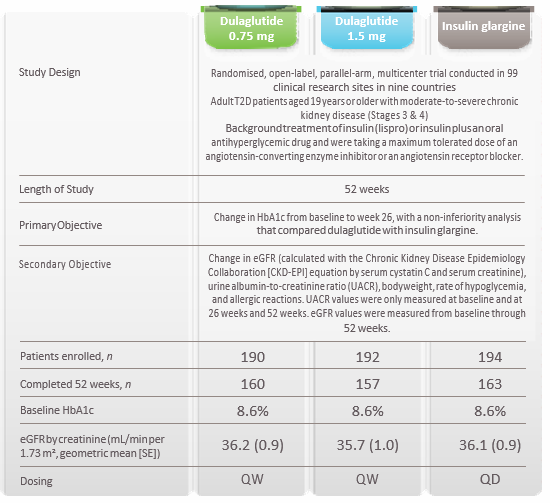
Results
In patients with moderate-to-severe chronic kidney disease, HbA1c reduction was comparable to insulin glargine and sustained at 52 weeks
HbA1c change from baseline (26 and 52 weeks)*
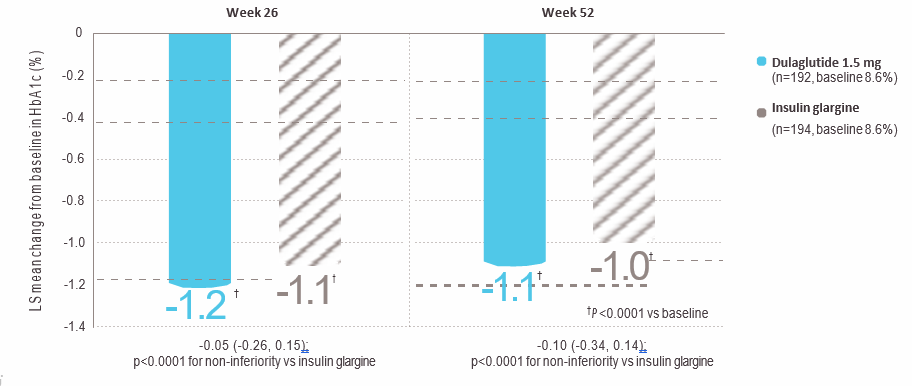
HbA1c=glycated haemoglobin*Graph adapted from Tuttle KR, et al. The Lancet Diabetes & Endocrinology. 2018. doi: 10.1016/S2113-8557(18)30104-9.No dosage adjustment required in patients with mild, moderate, or severe chronic kidney disease taking dulaglutide.
Dulaglutide was associated with smaller eGFR decline in people with T2D and moderate to severe chronic kidney disease compared to insulin glargine.eGFR change from baseline to 26 weeks*
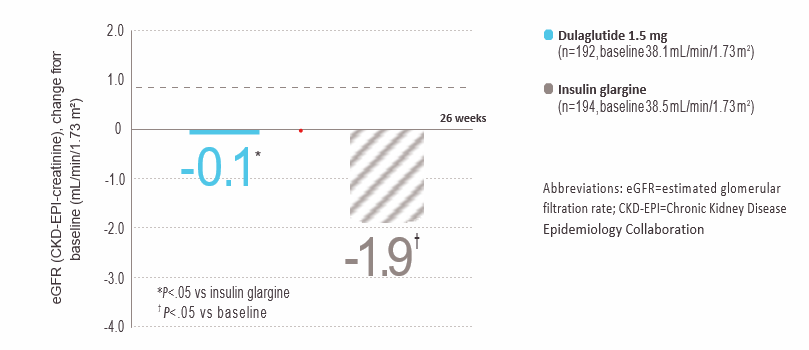
*Graph adapted from Tuttle KR, et al. The Lancet Diabetes & Endocrinology. 2018. doi: 10.1016/S2113-8557(18)30104-9.
Dulaglutide had lower rates of hypoglycemia compared to insulin glargine.Patients with hypoglycemia data Total hypoglycemiaProportion of patients ,
1-year adjusted rate, events/patient/year
Documented symptomatic hypoglycemiaIncidence ,
1-year adjusted rate, events/patient/year
Nocturnal hypoglycemiaProportion of patients
1-year adjusted rate, events/patient/year
Severe hypoglycemiaProportion of patients
1-year adjusted rate, events/patient/year
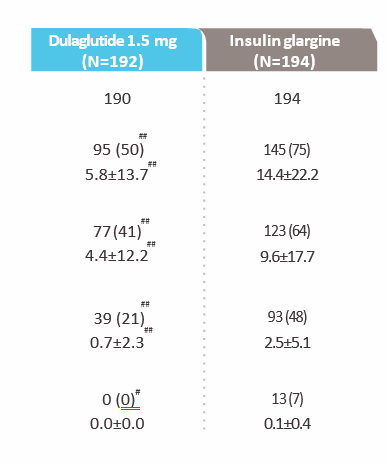
Data are n (%) (incidence) or mean±SD (rate); safety population; #p<0.05 and ##p<0.0001 vs insulin glargine. Documented symptomatic hypoglycemia was defined as any time a patient felt that he/she was experiencing symptoms and/or signs associated with hypoglycemia, and had a plasma glucose level of ≤70 mg/dL (3.9 mmol/L). Severe hypoglycemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Nocturnal hypoglycemia was defined as any hypoglycemic event that occurred between bedtime and waking and probable symptomatic hypoglycemia was defined as an event during which symptoms of hypoglycemia were not accompanied by a plasma glucose determination (but that was presumably caused by a plasma glucose level of ≤70 mg/dL [3.9 mmol/L]).

ConclusionsThe AWARD-7 study showed that the once-weekly dulaglutide treatment offers, besides a favourable safety profile, similar glycemic efficacy to daily insulin glargine treatment as basal therapy for patients with T2D and moderate-to-severe chronic kidney disease who had not previously attained glycemic targets with insulin alone or insulin plus oral antihyperglycemic agents. Therefore, dulaglutide treatment seems to have a favourable safety profile to achieve glycemic control in this patient populationReferences:1. Tuttle KR, et al. Lancet Diabetes & Endocrinology. 2018; doi: 10.1016/S2113-8587(18)30104−9.2. International Diabetes Federation. IDF Diabetes Atlas, 8th ed. Brussels, Belgium: International Diabetes Federation, 2017. 3. Stratton IM, et al. BMJ. 2000;321(7258): 405−412.4. World Health Organization. Adherence to long-term therapies – evidence for action. 2003 WHO website… http://apps.who.int/medicinedocs/en/d/Js4883e/6.html. Accessed 7 November 2017.5. Khunti S, et al. British Journal of Diabetes and Vascular Disease. 2015;15(2): 65−69.6. Umpierrez G, et al. Diabetes Care. 2014;37: 2168−2176.7. Nauck M, et al. Diabetes Care. 2014;37: 2149−2158. 8. Dungan KM, et al. Lancet. 2014;384: 1349−1357.9. Dungan KM, et al. Diabetes, Obesity and Metabolism. 2016;18: 475−482.10. Wysham C, et al. Diabetes Care. 2014;37: 2159−2167.11. Giorgino F, et al. Diabetes Care. 2015;38: 2241−2249.12. Ludvik B, et al. Lancet Diabetes & Endocrinology. 2018;6: 370−381. 13. Blonde L, et al. Lancet. 2015;385: 2057−2066.14. Pozzilli P, et al. Diabetes, Obesity and Metabolism. 2017;19: 1024−1031.15. Bailey C and Day C. British Journal of Diabetes & Vascular Disease. 2012;12: 167−171,
PP-DG-SA-0558
Prescribing Information
© 2020 Eli Lilly and Company. All rights reserved.